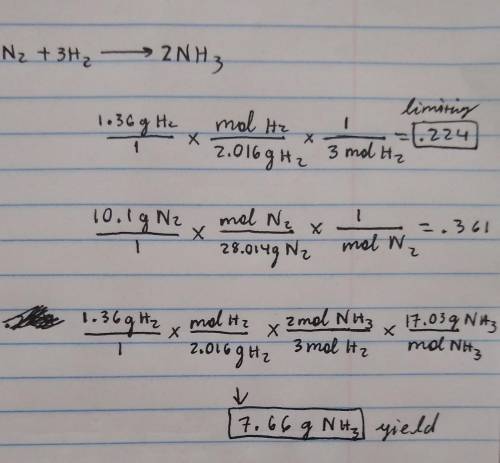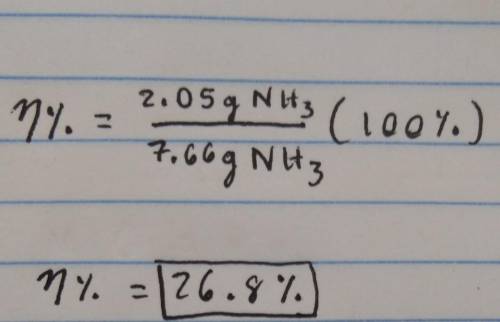Chemistry, 18.07.2021 22:10, calthecrystal
1.36g H2 is allowed to react with 10.1g N2 producing 2.05g NH3 What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
1.36g H2 is allowed to react with 10.1g N2 producing 2.05g NH3 What is the theoretical yield in gram...
Questions in other subjects:

Mathematics, 04.01.2020 07:31





Social Studies, 04.01.2020 07:31

Social Studies, 04.01.2020 07:31

Social Studies, 04.01.2020 07:31