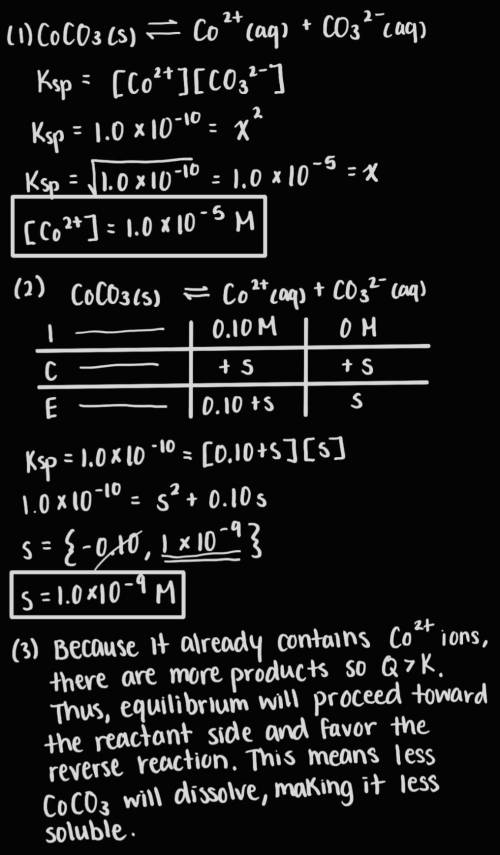Chemistry, 09.04.2021 23:50, valoiserika1229
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1.0 × 10^−10.
A. Calculate the value of [Co2+] in a saturated solution of CoCO3 in distilled water.
B. If 0.10 M of Co2+ is already present in distilled water, calculate the molar solubility of CoCO3(s).
C. Explain why CoCO3 is less soluble in distilled water that already contains Co2+

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
Do you know the correct answer?
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1....
Questions in other subjects:


Mathematics, 01.03.2021 08:50




Mathematics, 01.03.2021 08:50


Mathematics, 01.03.2021 08:50

Mathematics, 01.03.2021 08:50

English, 01.03.2021 08:50


![K = \frac{[products]}{[reactants]}](/tpl/images/1250/0658/0c10f.png) increases.
increases.