=
Breanna
Combustion analysis
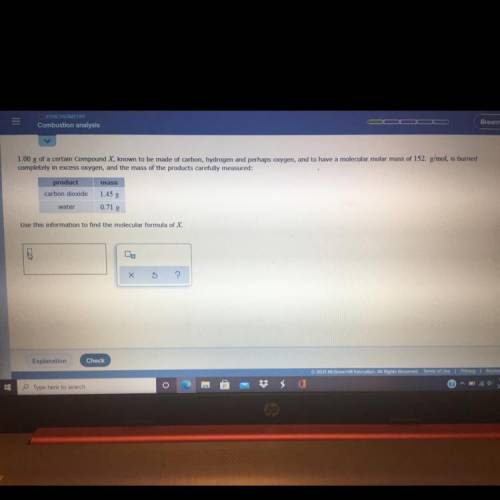
1.00 g of a certain Compound X, known to be made of carbo...

=
Breanna
Combustion analysis
1.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 152. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
mass
product
carbon dioxide
1.45 g
0.71 g
water
do
Use this information to find the molecular formula of X
B
Х
5
?
Explanation
Check
2021 McGraw He Education. All Rights Reserved. Terms of Use Privacy | Access
11:35 PM
() Ap司
2/28/2021
O
Type here to search

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 21:30, rondonalba
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


English, 28.01.2021 07:20

Mathematics, 28.01.2021 07:20

History, 28.01.2021 07:20

Mathematics, 28.01.2021 07:20

History, 28.01.2021 07:20










