
Chemistry, 01.03.2021 08:50, ZayBoogie4771
NEED HELP QUICK! 100 POINTS! Also need this solved Calculate the number of moles of Al required to produce 76.37 moles of H2. Do not include units in your answer. Round to the tenths place or further.
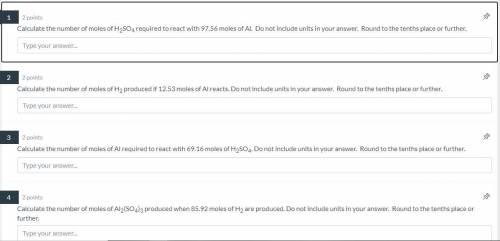

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
Do you know the correct answer?
NEED HELP QUICK! 100 POINTS!
Also need this solved Calculate the number of moles of Al required to...
Questions in other subjects:


Physics, 04.07.2019 00:30



Mathematics, 04.07.2019 00:30


Mathematics, 04.07.2019 00:30

Mathematics, 04.07.2019 00:30








