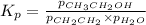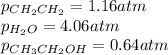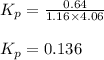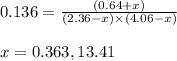Chemistry, 18.03.2020 22:08, kellinvagneur
While ethanol is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it is synthesized by reacting ethylene with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a flask with of ethylene gas and of water vapor. When the mixture has come to equilibrium she determines that it contains of ethylene gas and of water vapor. The engineer then adds another of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
Do you know the correct answer?
While ethanol is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it...
Questions in other subjects:










Mathematics, 07.04.2020 01:35


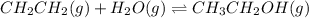
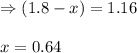
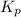 for above equation follows:
for above equation follows: