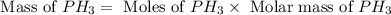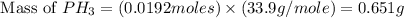Chemistry, 07.04.2020 01:35, harodkdc7910
The reaction of NH3 and O2 forms NO and water. The NO can be used to convert P4 to P4O6, forming N2 in the process. The P4O6 can be treated with water to form H3PO3, which forms PH3 and H3PO4 when heated. Find the mass of PH3 that forms from the reaction of 1.95 g NH3.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
Do you know the correct answer?
The reaction of NH3 and O2 forms NO and water. The NO can be used to convert P4 to P4O6, forming N2...
Questions in other subjects:


Mathematics, 28.04.2021 01:00

Biology, 28.04.2021 01:00

Geography, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

Health, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

English, 28.04.2021 01:00


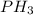 produced from the reaction is, 0.651 grams.
produced from the reaction is, 0.651 grams.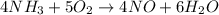
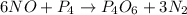
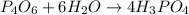
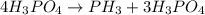
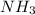

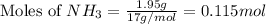
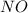
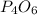
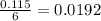 moles of
moles of 
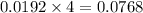 moles of
moles of 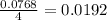 moles of
moles of