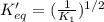Chemistry, 02.12.2019 21:31, heatherswiffin666
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k value for the reaction: so3(g) --> so2(g) + ½ o2(g)?
k1^½
1/k1
½ k1
(1/k1)^½

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
Do you know the correct answer?
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k va...
Questions in other subjects:


Mathematics, 16.10.2020 03:01

World Languages, 16.10.2020 03:01

Computers and Technology, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01





World Languages, 16.10.2020 03:01


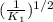
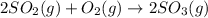
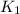
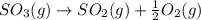
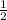 ', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.
', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.