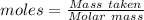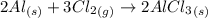Chemistry, 22.10.2019 03:30, carterh166
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(> 2alcl3(s) you are given 18.0g of aluminum and 23.0g of chlorine gas. part a: if you had excess chlorine, how many moles of of aluminum chloride could be produced from 18.0g of aluminum? express your answer numerically in moles. part b: if you had excess aluminum, how many moles of aluminum chloride could be produced from 23.0g of chlorine gas, cl2? express your answer numerically in moles.

Answers: 2
Similar questions

Chemistry, 21.06.2019 16:20, maevemboucher78
Answers: 2

Chemistry, 06.07.2019 01:10, kittykat8317
Answers: 1


Chemistry, 20.09.2019 01:10, macp98463oz47dl
Answers: 3
Do you know the correct answer?
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2...
Questions in other subjects:

Mathematics, 23.10.2020 18:50




Chemistry, 23.10.2020 18:50

Mathematics, 23.10.2020 18:50


Mathematics, 23.10.2020 18:50

Mathematics, 23.10.2020 18:50