
Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2...
Questions in other subjects:

Mathematics, 21.11.2020 01:40


Mathematics, 21.11.2020 01:40


Mathematics, 21.11.2020 01:40




Biology, 21.11.2020 01:40


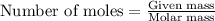 ....(1)
....(1) 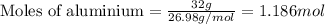
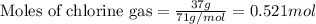
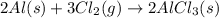
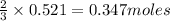 of aluminium.
of aluminium.




