
Chemistry, 31.07.2019 21:30, michellemitchel
At a certain temperature, kc = 0.0500 and ∆h = +39.0 kj for the reaction below, 2 mgcl2(s) + o2(g) → 2mgo(s) + cl2(g) calculate kc for the reaction, mgo(s) + ½ cl2(g) → mgcl2(s) + ½ o2(g) and indicate whether the value of kc will be larger or smaller at a lower temperature.

Answers: 1
Similar questions

Physics, 21.07.2019 19:30, marlesly87
Answers: 1



Chemistry, 23.10.2019 00:00, chycooper101
Answers: 3
Do you know the correct answer?
At a certain temperature, kc = 0.0500 and ∆h = +39.0 kj for the reaction below, 2 mgcl2(s) + o2(g) →...
Questions in other subjects:


Mathematics, 21.10.2020 01:01



Social Studies, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01

English, 21.10.2020 01:01


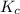 will become
will become 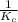 .
.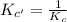
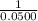
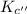 for the reaction
for the reaction 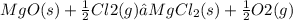 will also get halved.
will also get halved.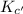 =
= 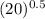
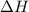 is given as +39.0 kJ. So, it means that the reaction is endothermic in nature. So, energy of reactants will be more than the products. Hence, according to Le Chatelier's principle reaction will move in the forward direction.
is given as +39.0 kJ. So, it means that the reaction is endothermic in nature. So, energy of reactants will be more than the products. Hence, according to Le Chatelier's principle reaction will move in the forward direction.



