Chemistry, 04.08.2019 21:30, cutiecat66
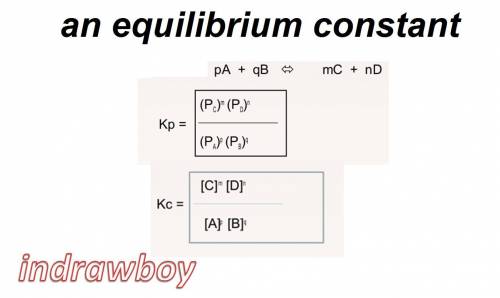
Given the information for reaction 1 at a specific temperature; calculate kc for reaction 2 at the same temperature. reaction 1: 2ch4 (g) ⇄ c2h2 (g) + 3h2 (g) kc = 0.020 reaction 2: 2c2h2 (g) + 6h2 (g) ⇄ 4ch4 (g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
Do you know the correct answer?
Given the information for reaction 1 at a specific temperature; calculate kc for reaction 2 at the...
Questions in other subjects:




History, 28.11.2019 03:31

Chemistry, 28.11.2019 03:31



Mathematics, 28.11.2019 03:31



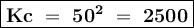

![\large{\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0170/8798/48dd8.png)
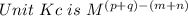
![\large{\boxed{\bold{Kc~=~\frac{[C_2H_2][H_2]^3}{[CH_4]^2} }}}](/tpl/images/0170/8798/ccc7e.png)
![\large{\boxed{\bold{Kc~=~\frac{[CH_4]^2}{[[C_2H_2][H_2]^3} }}}](/tpl/images/0170/8798/ae421.png)
![\large{\boxed{\bold{Kc~=~\frac{[CH_4]^4}{[[C_2H_2]^2[H_2]^6} }}}](/tpl/images/0170/8798/48299.png)