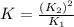Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Do you know the correct answer?
Calculate the equilibrium constant for the following reaction: 2cl2(g) + 2h2o(g) ⇌ 4hcl(g) + o2(g)...
Questions in other subjects:

Biology, 30.08.2020 15:01


History, 30.08.2020 15:01

Chemistry, 30.08.2020 15:01

Mathematics, 30.08.2020 15:01

Mathematics, 30.08.2020 15:01

English, 30.08.2020 15:01

Mathematics, 30.08.2020 15:01



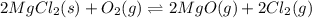

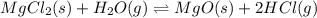

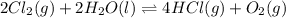

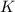 for the final reaction.
for the final reaction.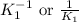 and multiplying the equation 2 by 2 that means we are writing the equilibrium constant as
and multiplying the equation 2 by 2 that means we are writing the equilibrium constant as 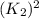 , we get the final equilibrium reaction and the expression of final equilibrium constant is:
, we get the final equilibrium reaction and the expression of final equilibrium constant is: