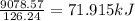Chemistry, 25.07.2019 20:20, NatalieAllen11
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) 12o2(g) → 5b2o3(s) 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1
Similar questions

Chemistry, 24.07.2019 12:30, milkshakegrande101
Answers: 2


Chemistry, 09.10.2019 03:30, fancycar14
Answers: 2

Chemistry, 30.10.2019 05:31, ayeeeee98
Answers: 1
Do you know the correct answer?
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid t...
Questions in other subjects:

English, 28.09.2019 20:10


History, 28.09.2019 20:10

World Languages, 28.09.2019 20:10





English, 28.09.2019 20:10


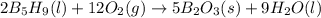
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0132/3420/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{B_2O_3}\times \Delta H_{B_2O_3})]-[(n_{B_5H_9}\times \Delta H_{B_5H_9})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0132/3420/48532.png)
![\Delta H=[(9\times -285.83)+(5\times -1271.94)]-[(2\times 73.2)+(12\times 0)]\\\\\Delta H=-9078.57kJ](/tpl/images/0132/3420/9a745.png)
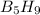 has 63.12 grams of mass
has 63.12 grams of mass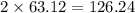 grams of mass
grams of mass