
Chemistry, 09.10.2019 03:30, fancycar14
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed to oxygen. the reaction is 2b5h9(l) + 12o2(g) ⟶ 5b2o3(s) + 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formation of b5h9 is 73.2 kj/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
Do you know the correct answer?
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed...
Questions in other subjects:


Mathematics, 17.10.2021 21:10


History, 17.10.2021 21:20


Mathematics, 17.10.2021 21:20


History, 17.10.2021 21:20

Business, 17.10.2021 21:20

Mathematics, 17.10.2021 21:20


 is -71.92 kJ
is -71.92 kJ
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0302/4270/72c39.png)
![\Delta H^o_{rxn}=[(5\times \Delta H^o_f_{(B_2O_3(s))})+(9\times \Delta H^o_f_{(H_2O(l))})]-[(2\times \Delta H^o_f_{(B_5H_9(l))})+(12\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0302/4270/e310e.png)
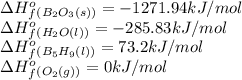
![\Delta H^o_{rxn}=[(5\times (1271.94))+(9\times (-285.83))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H^o_{rxn}=-9078.57kJ](/tpl/images/0302/4270/015ae.png)
 of
of 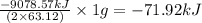 of energy.
of energy.



