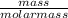Chemistry, 02.11.2019 23:31, pgjohnston001
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (iii) sulfide in moles would be produced in this reaction?
equation:
convert to moles
of iron:
convert to moles
of sulfur:
calculate the
limiting reagent:
solve the problem:

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
Do you know the correct answer?
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (ii...
Questions in other subjects:

Social Studies, 26.03.2020 17:34


Mathematics, 26.03.2020 17:34



Biology, 26.03.2020 17:34

Social Studies, 26.03.2020 17:34