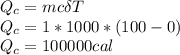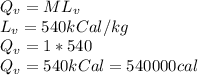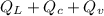The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:T, where c is the specific heat capacity of the substance. Forexample, forH20,c=1caljg'C",Andfora change of phase, the quantity of heat Q that changes the phase of a mass m is Q = ml., where L is the heat of fusion or heat of vaporization of the substance. For example, for H20, the heat offusion is 80 cal/g (or 80 kcaljkg) and the heat of vaporization is 540 cal/g (or 540 kcaljkg). Use these relationships to determine the number of calories to change (a) 1 kg ofO°C ice to O°C ice water, (b) 1 kg ofO°C ice water to 1 kg of 100°C boiling water, (c) 1 kg of 100°C boiling water to 1 kg of 100°C steam, and (d) 1 kg ofO°C ice to 1 kg of 100°C steam.

Answers: 3
Other questions on the subject: Physics



Physics, 22.06.2019 10:10, sadie960
Aclarinetist, setting out for a performance, grabs his 3.350 kg clarinet case (including the clarinet) from the top of the piano and carries it through the air with an upward force of 24.65 n. find the case's vertical acceleration. indicate an upward acceleration as positive and a downward one as negative.
Answers: 1

Do you know the correct answer?
The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:...
Questions in other subjects:











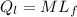 = 1 * 80
= 1 * 80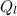 = 80 kCal = 80,000 cal
= 80 kCal = 80,000 cal