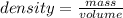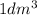Chemistry, 14.08.2019 08:10, BandNerd922
The number of atoms in 1 dm3 of aluminum is nearly the same as the number of atoms in 1 dm3 of lead, but the densities of these metals are very different (see table 1.5). explain

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
Do you know the correct answer?
The number of atoms in 1 dm3 of aluminum is nearly the same as the number of atoms in 1 dm3 of lead,...
Questions in other subjects:




Chemistry, 19.01.2020 06:31

Physics, 19.01.2020 06:31

English, 19.01.2020 06:31



Mathematics, 19.01.2020 06:31