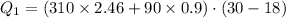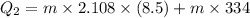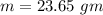Physics, 14.02.2020 19:52, chanavictor2747
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are at 30.0C. A mass m of ice at – 8.5C is taken from a freezer and added to the alcohol in the cup. The final temperature of all the components is 18.0C. Assuming no heat was lost from the system, calculate the mass m of the ice added.

Answers: 2
Other questions on the subject: Physics


Physics, 22.06.2019 15:20, avree4722
Abag of potato chips contains 2.00 l of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0 deg c. what will be the volume of the air in the bag if you take it with you, still sealed, to the mountains where the temperature is 7.00 deg c and atmospheric pressure is 70.0 kpa? assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air.
Answers: 3

Physics, 22.06.2019 21:30, tashaylinm02
Which sections of the heating curve illustrate this process?
Answers: 2

Physics, 22.06.2019 22:00, shahrukhafridi2668
Which best term to describe a chemical reaction that absorbs energy from its surroundings? a. thermal conductor b. thermal insulator c. endothermic d. exothermic
Answers: 1
Do you know the correct answer?
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are...
Questions in other subjects:

Mathematics, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00



Physics, 13.11.2020 01:00




Arts, 13.11.2020 01:00

Mathematics, 13.11.2020 01:00


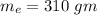
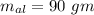
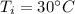
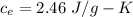
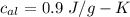
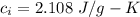
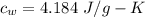
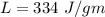
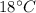 is reached
is reached