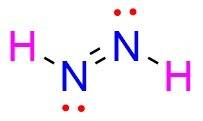
Chemistry, 23.07.2019 16:30, fashionblogger28
The lewis structure of n2h2 shows a. a nitrogen-nitrogen triple bond b. a nitrogen-nitrogen single bond c. each nitrogen has one nonbinding electron pair d. each nitrogen has two nonbinding electron pairs e. each hydrogen has one nonbonding electron pair

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
Do you know the correct answer?
The lewis structure of n2h2 shows a. a nitrogen-nitrogen triple bond b. a nitrogen-nitrogen single...
Questions in other subjects:



Mathematics, 15.01.2020 04:31


History, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31



Mathematics, 15.01.2020 04:31