
Chemistry, 28.07.2019 04:00, aleyshamar14p95561
Co(g) + 2 h2 --> ch3oh 2.50 g of hydrogen is reacted with 30.0 l of carbon monoxide at stp. 1. what is the limiting reactant? *hint: only list the element symbol* 2. what mass of ch3oh is produced? *hint: only list the grams* 3. how much excess is left over? *hint: only list the grams*

Answers: 1
Similar questions


Chemistry, 12.07.2019 05:30, lilinicholeb
Answers: 1

Chemistry, 02.08.2019 05:30, samanthablain192
Answers: 2

Chemistry, 08.08.2019 06:20, lilyrockstarmag
Answers: 3
Do you know the correct answer?
Co(g) + 2 h2 --> ch3oh 2.50 g of hydrogen is reacted with 30.0 l of carbon monoxide at stp. 1. w...
Questions in other subjects:


History, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00



Chemistry, 27.06.2019 22:00

History, 27.06.2019 22:00

Biology, 27.06.2019 22:00



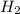
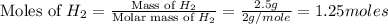
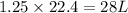 volume of hydrogen gas
volume of hydrogen gas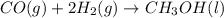
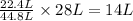 of carbon monoxide gas
of carbon monoxide gas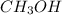 is, 20 grams
is, 20 grams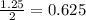 mole of
mole of 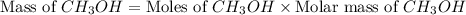
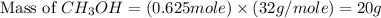
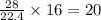 gram of carbon monoxide gas
gram of carbon monoxide gas




