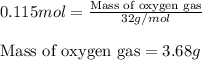Chemistry, 08.08.2019 06:20, lilyrockstarmag
Identify the limiting reactant in the reaction of carbon monoxide and oxygen to form co2, if 9.16 g of co and 9.01 g of o2 are combined. determine the amount (in grams) of excess reactant that remains after the reaction is complete.
formula of limiting reactant =
amount of excess reactant remaining = g

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Do you know the correct answer?
Identify the limiting reactant in the reaction of carbon monoxide and oxygen to form co2, if 9.16 g...
Questions in other subjects:



Health, 22.07.2021 21:20


English, 22.07.2021 21:20

Mathematics, 22.07.2021 21:20




Mathematics, 22.07.2021 21:20


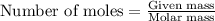 ....(1)
....(1) 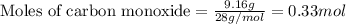
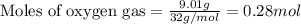
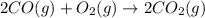
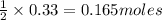 of oxygen gas
of oxygen gas