

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Do you know the correct answer?
Compared to the freezing point of 1.0 m kcl(aq) at standard pressure, the freezing point of 1.0 m ca...
Questions in other subjects:



Mathematics, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50


Mathematics, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50



Mathematics, 19.03.2021 08:50


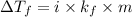
 = change in freezing point
= change in freezing point
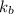 = freezing point constant
= freezing point constant
 will be,
will be,
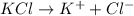
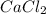 will be,
will be,
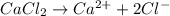
 at standard pressure, the freezing point of 1.0 M
at standard pressure, the freezing point of 1.0 M  at standard pressure is lower.
at standard pressure is lower.



