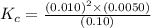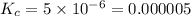Chemistry, 02.08.2019 22:30, aprilreneeclaroxob0c
For the reaction 2nobr (g) ↔ 2no (g) + br2 (g), when the concentrations are [nobr] = 0.10 m, [no] = 0.010 m, and [br2] = 0.0050 m, what is the equilibrium constant of this reaction? a. 5.0 × 10–5 m b. 0.000005 m c. 5.0 × 105 m d. 5.0 m e. none of the above

Answers: 1
Similar questions

Chemistry, 25.07.2019 14:00, glocurlsprinces
Answers: 1

Chemistry, 31.08.2019 00:00, hmatt5643
Answers: 1

Chemistry, 12.09.2019 19:30, lolaloiuy7695
Answers: 3

Chemistry, 01.10.2019 22:00, manarsadi6
Answers: 2
Do you know the correct answer?
For the reaction 2nobr (g) ↔ 2no (g) + br2 (g), when the concentrations are [nobr] = 0.10 m, [no] =...
Questions in other subjects:


World Languages, 16.10.2019 22:00



Mathematics, 16.10.2019 22:00

Mathematics, 16.10.2019 22:00





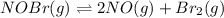
![K_c=\frac{[NO]^2[Br_2]}{[NOBr]}](/tpl/images/0163/3611/32da8.png)