
Chemistry, 01.10.2019 22:00, manarsadi6
The decomposition of nobr is studied manometrically because the number of moles of gas changes; it cannot be studied colorimetrically because both nobr and br2 are reddish-brown. 2nobr(g) →2no(g) + br2(g) use the data below to make the following determinations: (a) the average rate of decomposition of nobr over the entire experiment. (b) the average rate of decomposition of nobr between 2.00 and 4.00 seconds. time (s) [nobr] (mol/l)0.00 0.01002.00 0.00714.00 0.00556.00 0.00458.00 0.003810.00 0.0033the rates of decomposition of nobr are

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 07:00, ultimatesaiyan
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
Do you know the correct answer?
The decomposition of nobr is studied manometrically because the number of moles of gas changes; it...
Questions in other subjects:






Mathematics, 26.02.2021 20:20

Physics, 26.02.2021 20:20


Mathematics, 26.02.2021 20:20


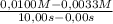 = 6,7x10⁻⁴M/s
= 6,7x10⁻⁴M/s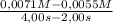 = 8,0x10⁻⁴M/s
= 8,0x10⁻⁴M/s




