Chemistry, 19.07.2019 17:20, Raekwon3232
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n2o4 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 = 92.02 g/mol, n2h4 = 32.05 g/mol. n2o4(l) + 2 n2h4(l) → 3 n2(g) + 4 h2o(g) determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n2o4 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 = 92.02 g/mol, n2h4 = 32.05 g/mol. n2o4(l) + 2 n2h4(l) → 3 n2(g) + 4 h2o(g) lr = n2o4, 105 g n2 formed lr = n2h4, 59.0 g n2 formed lr = n2o4, 45.7 g n2 formed no lr, 45.0 g n2 formed lr = n2h4, 13.3 g n2 formed

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
Do you know the correct answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions in other subjects:


Mathematics, 09.12.2020 06:50









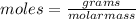 (molar mass of N2= 28)
(molar mass of N2= 28)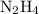 . The moles of nitrogen formed has been 2.1 mol of nitrogen.
. The moles of nitrogen formed has been 2.1 mol of nitrogen.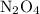 and
and