
Chemistry, 10.07.2019 20:50, shekinahdavis8760
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17%. 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%. show your work.

Answers: 1
Similar questions

Chemistry, 10.07.2019 00:00, Officialavm
Answers: 1

Chemistry, 27.08.2019 07:00, Jerrikasmith28
Answers: 1

Chemistry, 30.09.2019 22:30, kayleigh2037
Answers: 3
Do you know the correct answer?
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an...
Questions in other subjects:





History, 03.02.2020 21:02

Mathematics, 03.02.2020 21:02

English, 03.02.2020 21:02


Mathematics, 03.02.2020 21:02

Spanish, 03.02.2020 21:02


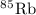 has atomic mass = 84.912 amu
has atomic mass = 84.912 amu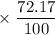
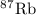 has atomic mass = 86.909 amu
has atomic mass = 86.909 amu




