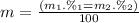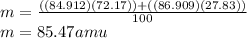Chemistry, 27.08.2019 07:00, Jerrikasmith28
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.
note that 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17% while 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%.

Answers: 1
Similar questions

Chemistry, 10.07.2019 00:00, Officialavm
Answers: 1

Chemistry, 10.07.2019 20:50, shekinahdavis8760
Answers: 1

Chemistry, 30.09.2019 22:30, kayleigh2037
Answers: 3
Do you know the correct answer?
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.<...
Questions in other subjects:

History, 28.10.2019 18:31

Mathematics, 28.10.2019 18:31


Physics, 28.10.2019 18:31






Mathematics, 28.10.2019 18:31