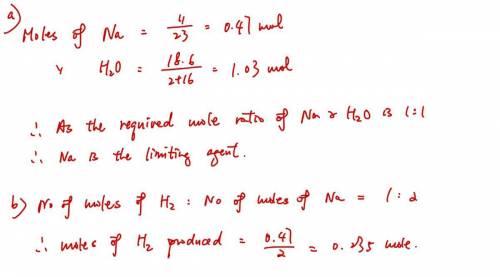A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydrogen gas. Using the balanced equation below, predict which is the limiting reactant and the maximum amount in moles of hydrogen gas that can be produced. 2Na+ 2H2O + 2NaOH + H2

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
Do you know the correct answer?
A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydroge...
Questions in other subjects:

Computers and Technology, 15.12.2020 18:10



Mathematics, 15.12.2020 18:10



History, 15.12.2020 18:10



Mathematics, 15.12.2020 18:10