Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
Do you know the correct answer?
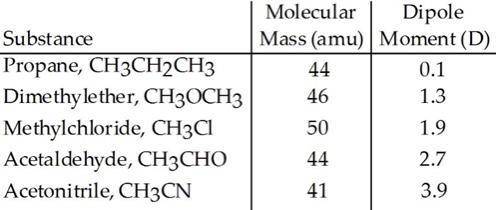
Based on molecular mass and dipole moment of the five compounds in the table below, which should hav...
Questions in other subjects:


History, 22.06.2019 23:00





Mathematics, 22.06.2019 23:00


Geography, 22.06.2019 23:00

History, 22.06.2019 23:00