
Chemistry, 04.08.2021 15:50, aidenmanpig
Briefly workout the relationship between these constants:
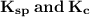
In consideration of the decopmposition of hydrogen iodide.
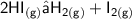

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
Briefly workout the relationship between these constants:
In consideration of the decopmposit...
In consideration of the decopmposit...
Questions in other subjects:

Mathematics, 30.11.2021 06:30



Mathematics, 30.11.2021 06:30

Physics, 30.11.2021 06:30

Mathematics, 30.11.2021 06:30

History, 30.11.2021 06:30



History, 30.11.2021 06:30






