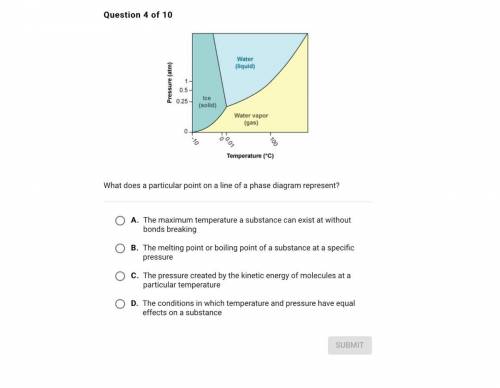
Question 4 of 10
Water
(liquid)
Pressure (atm)
1
0.5-
0.25
Ice<...

Question 4 of 10
Water
(liquid)
Pressure (atm)
1
0.5-
0.25
Ice
(solid)
Water vapor
(gas)
0
160.01
-10
100
Temperature (°C)
What does a particular point on a line of a phase diagram represent?
A. The maximum temperature a substance can exist at without
bonds breaking
B. The melting point or boiling point of a substance at a specific
pressure
C. The pressure created by the kinetic energy of molecules at a
particular temperature
D. The conditions in which temperature and pressure have equal
effects on a substance

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Social Studies, 24.06.2021 17:20


Mathematics, 24.06.2021 17:20

Geography, 24.06.2021 17:20

Mathematics, 24.06.2021 17:20


Mathematics, 24.06.2021 17:20

Mathematics, 24.06.2021 17:20






