
Chemistry, 17.06.2021 17:20, emilyharper
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determine your percent error for both gas samples.
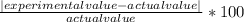
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe? (Hint: Choose one volume and temperature pair from your data table to use in your ideal gas law calculation.)
I literally have no clue what this is asking for. The graphs are "best-fit line" graphs that were made from the data table (all are attached including the instructions about making the graph)
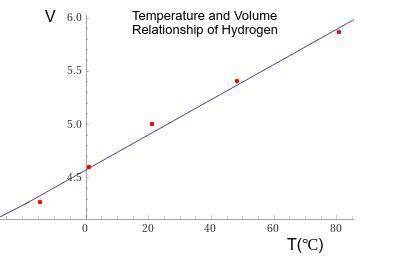
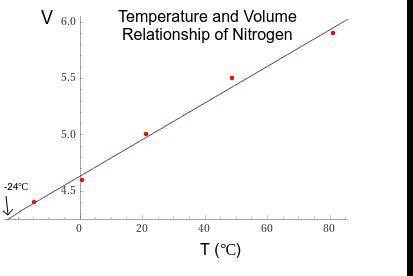
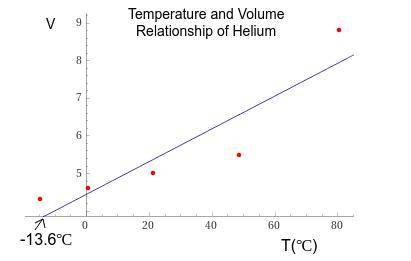
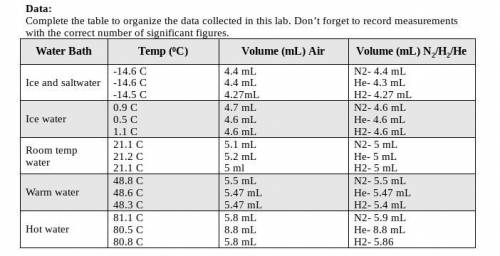
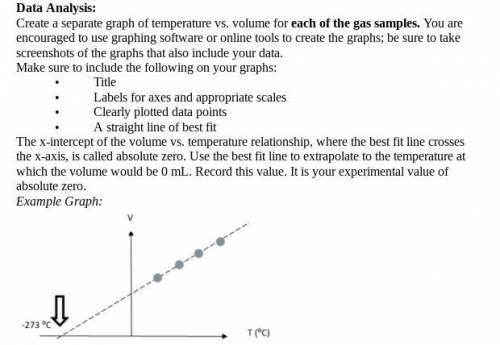

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 11:00, artiomtyler007
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
Do you know the correct answer?
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determ...
Questions in other subjects:



Mathematics, 04.08.2021 02:20






English, 04.08.2021 02:20

Mathematics, 04.08.2021 02:20






