
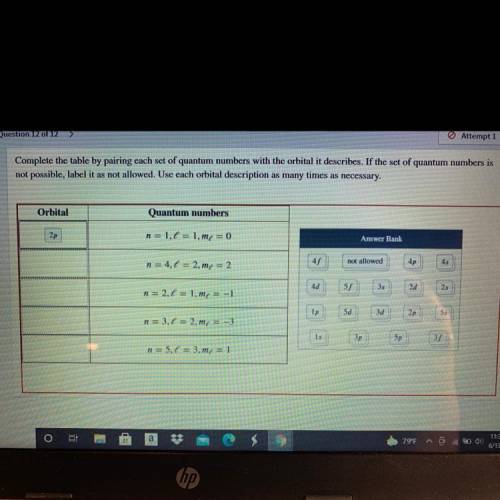
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is
not possible, label it as not allowed. Use each orbital description as many times as necessary.
Orbital
Quantum numbers
2p
n = 1.6 = 1.me = 0
Answer Bank
n = 4.1 = 2.m = 2
45
not allowed
4p
45
4d
n = 2.1 = 1.mx = -1
55
35
2d
25
Ip
5d
3d
2p
55
n = 3,6 = 2.mx = -3
1s
3p
5p
35
n = 5.0 = 3.m4 = 1
7
O
Bi
11:36 PM

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, hannah2718
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
Do you know the correct answer?
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set...
Questions in other subjects:















