1. Specific Heat Capacity.
A. Heat on concrete
The specific heat capacity of concrete is 0.88...

Chemistry, 03.06.2021 16:50, genyjoannerubiera
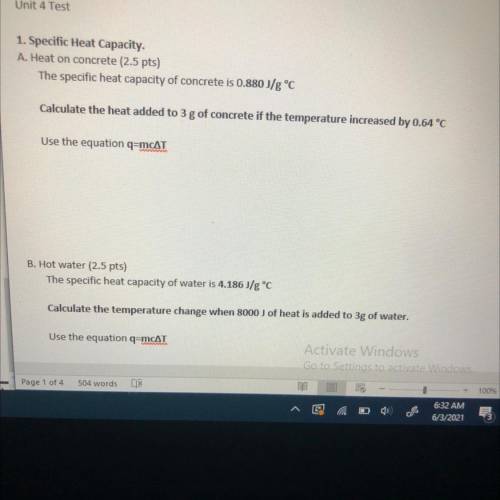
1. Specific Heat Capacity.
A. Heat on concrete
The specific heat capacity of concrete is 0.880 J/g °C
Calculate the heat added to 3 g of concrete if the temperature increased by 0.64 °C
Use the equation q=mcat
B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature change when 8000 J of heat is added to 3g of water,
Use the equation q=mcat

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 14:30, jenorajordan5387
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Mathematics, 09.09.2019 19:10

Social Studies, 09.09.2019 19:10

Physics, 09.09.2019 19:10


Mathematics, 09.09.2019 19:10

Biology, 09.09.2019 19:10










