Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Do you know the correct answer?
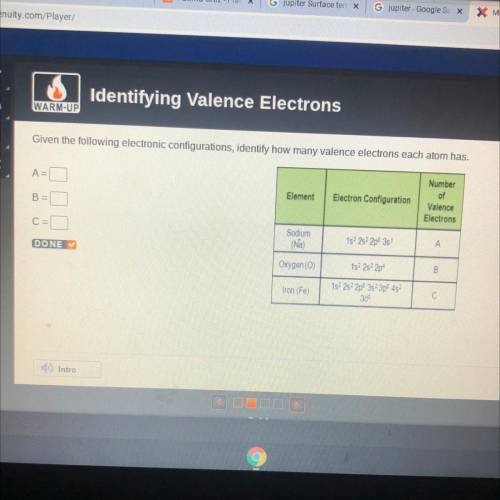
Given the following electronic configurations, identify how many valence electrons each atom has.
A...
Questions in other subjects:


Social Studies, 09.01.2020 06:31


Social Studies, 09.01.2020 06:31





Social Studies, 09.01.2020 06:31