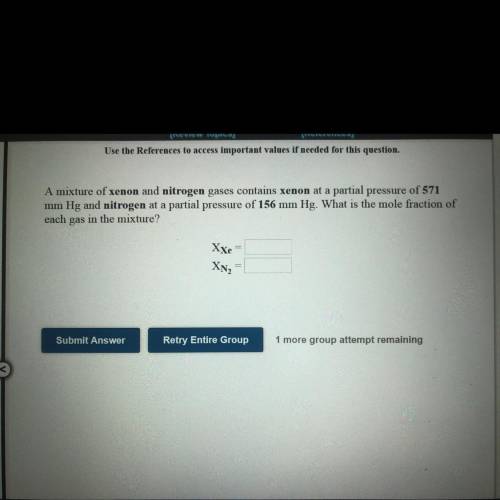
A mixture of xenon and nitrogen gases contains xenon at a partial pressure
of 571
mm Hg and...

Chemistry, 04.05.2021 06:10, chriscook4471
A mixture of xenon and nitrogen gases contains xenon at a partial pressure
of 571
mm Hg and nitrogen at a partial pressure of 156 mm Hg. What is the mole fraction of
each gas in the mixture?
Please look at the picture above
XXe=?
XN2=?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2
Do you know the correct answer?
Questions in other subjects:








Social Studies, 13.03.2020 16:20








