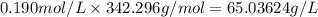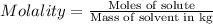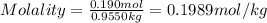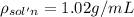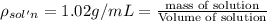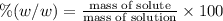Chemistry, 02.10.2019 12:30, irenecupcake4348
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of g/l, molality, and mass % (molecular weight, mwsucrose = 342.296 g/mol ; density, ρsol′n = 1.02 g/ml ; mass of water, mwat = 955.0 g ). note that the mass of solute is included in the density of the solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
Do you know the correct answer?
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of...
Questions in other subjects:

Mathematics, 28.01.2020 23:46

Mathematics, 28.01.2020 23:46







Mathematics, 28.01.2020 23:46