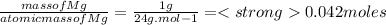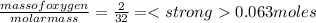Which statement is true about limiting reactants?
a). the limiting reactant in a reaction is the first reactant to be completely converted to moles.
b). the limiting reactant in a reaction is the first reactant to be completely converted to products.
c).the limiting reactant in a reaction is the first reactant to be completely converted to energy.
d).the limiting reactant in a reaction is the first reactant to be completely converted to heat.

Answers: 2
Similar questions


Chemistry, 24.10.2019 08:43, Samuelgamboe
Answers: 3

Chemistry, 25.10.2019 16:43, Josephcastillo6403
Answers: 2

Chemistry, 31.10.2019 17:31, imeldaa5009
Answers: 2
Do you know the correct answer?
Which statement is true about limiting reactants?
a). the limiting reactant in a reaction is...
a). the limiting reactant in a reaction is...
Questions in other subjects:

Physics, 13.11.2019 22:31



Mathematics, 13.11.2019 22:31




History, 13.11.2019 22:31