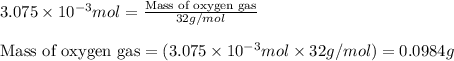Oxygen is produced by the reaction of sodium peroxide and water.
2na2o2(s) + 2h2o(l) > o2(g) + 4naoh(aq)
a. calculate the mass of na2o2 in grams needed to form 4.80g of oxygen.
b. how many grams of naoh are produced when 4.80 g of o2 is formed?
c. when 0.48g of na2o2 is dropped in water, how many grams of o2 are formed?

Answers: 1
Similar questions


Chemistry, 30.06.2019 06:30, kyledsmith18
Answers: 1

Chemistry, 14.07.2019 18:00, AleciaCassidy
Answers: 1

Chemistry, 27.07.2019 07:50, lauren21bunch
Answers: 2
Do you know the correct answer?
Oxygen is produced by the reaction of sodium peroxide and water.
2na2o2(s) + 2h2o(l) &g...
2na2o2(s) + 2h2o(l) &g...
Questions in other subjects:


Chemistry, 15.07.2021 06:20

Mathematics, 15.07.2021 06:20

Mathematics, 15.07.2021 06:20

Mathematics, 15.07.2021 06:20

Mathematics, 15.07.2021 06:20

History, 15.07.2021 06:20



English, 15.07.2021 06:30


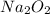 required is 23.4 grams
required is 23.4 grams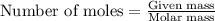 .....(2)
.....(2)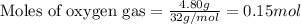
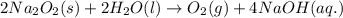
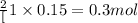 of
of 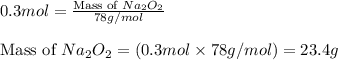
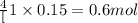 of NaOH will be produced.
of NaOH will be produced.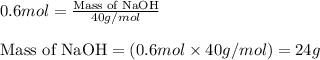
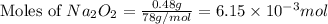
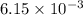 moles of
moles of 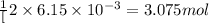 of oxygen gas
of oxygen gas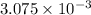 moles
moles