
Chemistry, 27.06.2019 03:20, xnadertheking
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the reaction of 10.0 g of na2o2 with water is kj. 2na2o2 (s) + 2h2o (l) → 4naoh (s) + o2 (g)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
Do you know the correct answer?
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the react...
Questions in other subjects:





Mathematics, 14.07.2020 14:01






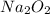 will be -8.064 kJ.
will be -8.064 kJ.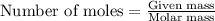
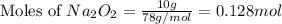
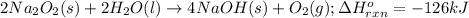
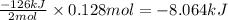 of energy.
of energy.




