
Chemistry, 14.10.2019 22:00, yeicooyola3
Ammonia is produced by the following reaction.
3h2(g) + n2(g) > 2nh3(g)
when 7.00 g of hydrogen react with 70.0 g of nitrogen, hydrogen is considered the limiting reactant because
7.5 mol of hydrogen would be needed to consume the available nitrogen.
7.5 mol of nitrogen would be needed to consume the available hydrogen.
hydrogen would produce 7.5 mol more ammonia than nitrogen.
nitrogen would produce 7.5 mol more ammonia than hydrogen.

Answers: 3
Similar questions


Chemistry, 25.07.2019 19:00, shoafmckenzie1962
Answers: 1

Chemistry, 30.09.2019 22:30, alyvia05
Answers: 2

Chemistry, 29.10.2019 19:31, snicklegirlp4isqr
Answers: 2
Do you know the correct answer?
Ammonia is produced by the following reaction.
3h2(g) + n2(g) > 2nh3(g)
...
3h2(g) + n2(g) > 2nh3(g)
...
Questions in other subjects:






Mathematics, 19.03.2020 21:09

Mathematics, 19.03.2020 21:09




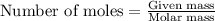
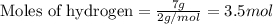
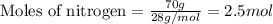
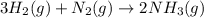
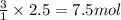 of nitrogen gas.
of nitrogen gas.




