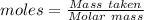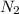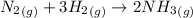Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, shahedalahmad2017
What is common about these molecules? a. their atoms are held together by covalent bonds. b. they are all made up of the same two atoms. c. their atoms are held together by ionic bonds. d. they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
Do you know the correct answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions in other subjects:

Mathematics, 21.04.2021 21:30

Mathematics, 21.04.2021 21:30

Mathematics, 21.04.2021 21:30



Mathematics, 21.04.2021 21:30



Mathematics, 21.04.2021 21:30

History, 21.04.2021 21:30


 .
.