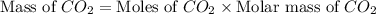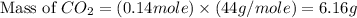You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bubbles as a reaction takes place. you then weigh the resulting solution and find that it has a mass of 64.96 g . the relevant equation is
caco3(s)+2hcl(aq)→h2o(l)+co2(g)+cac l2(aq)
assuming no other reactions take place, what mass of co2 was produced in this reaction?

Answers: 1
Similar questions

Chemistry, 30.07.2019 01:30, rainbowboi
Answers: 1

Chemistry, 01.09.2019 19:30, arjunchandrasek
Answers: 1

Chemistry, 06.10.2019 16:50, skaterwolf1317
Answers: 2
Do you know the correct answer?
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bu...
Questions in other subjects:






Mathematics, 19.08.2020 04:01





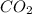 produced will be, 6.16 grams.
produced will be, 6.16 grams.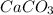 = 14 g
= 14 g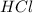 = 56.70 g
= 56.70 g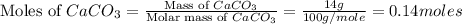
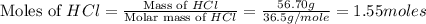
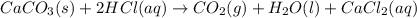
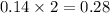 moles of
moles of