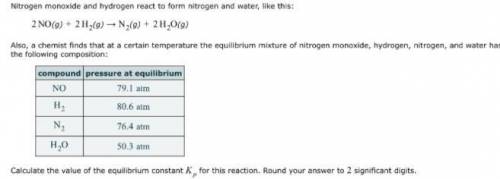
Nitrogen monoxide and hydrogen react to form nitrogen and water, like this: 2NO(g) 2H2(g) N2(g) 2H2O(g)Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen monoxide, hydrogen, nitrogen, and water has the following composition:compoundpressure at equilibriumCalculate the value of the equilibrium constant Kp for this reaction. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
Do you know the correct answer?
Nitrogen monoxide and hydrogen react to form nitrogen and water, like this: 2NO(g) 2H2(g) N2(g) 2H2O...
Questions in other subjects:



Computers and Technology, 20.07.2020 07:01





Mathematics, 20.07.2020 07:01

English, 20.07.2020 07:01