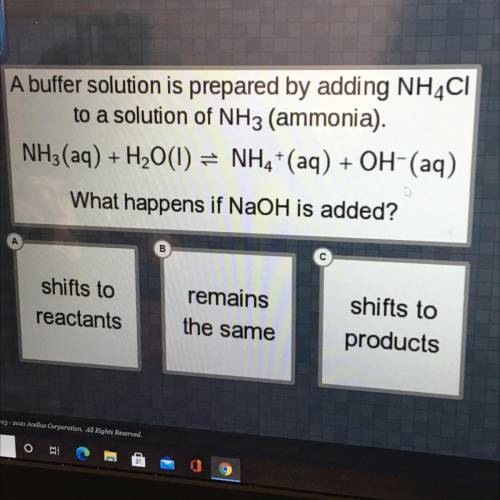
A buffer solution is prepared by adding NH4Cl
to a solution of NH3 (ammonia).
NH3(aq) + H2O(l...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
Do you know the correct answer?
Questions in other subjects:



Mathematics, 05.05.2020 02:15



Biology, 05.05.2020 02:15

Mathematics, 05.05.2020 02:15



Mathematics, 05.05.2020 02:15