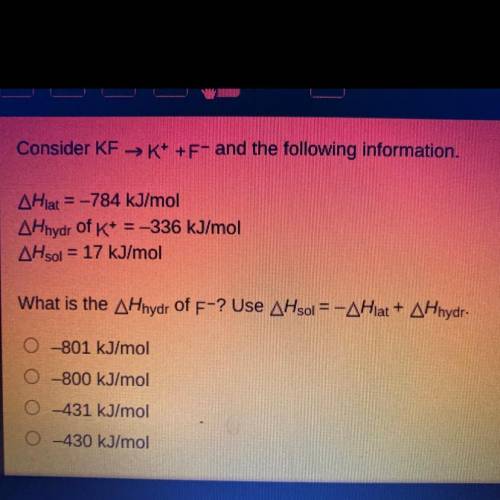
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ...

Chemistry, 02.04.2021 01:40, adelawilliams60
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ/mol
AHsol = 17 kJ/mol
What is the AHhydr of F-? Use AHsol = -AHlat + AHhydr.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3



Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Biology, 31.08.2019 00:00


Mathematics, 31.08.2019 00:00



Social Studies, 31.08.2019 00:00



Mathematics, 31.08.2019 00:00

History, 31.08.2019 00:00






