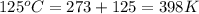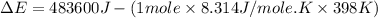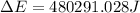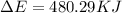Chemistry, 31.08.2019 00:00, nathaniel12
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are converted h2(g) and o2(g) against a pressure of 1 atm at 125 degrees celcius what is δe of reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are co...
Questions in other subjects:


Mathematics, 05.02.2021 22:40


Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40


Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40


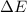 of the reaction is, 480.29 KJ.
of the reaction is, 480.29 KJ.
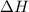 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J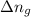 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole