7
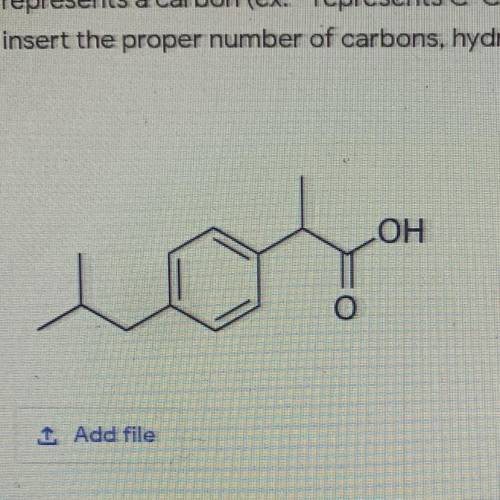
Apply your understanding of the OCTET rule and HONC-1234 to complete 5 points
the following...

7
Apply your understanding of the OCTET rule and HONC-1234 to complete 5 points
the following Lewis Structure. * remember the END of EVERY line
represents a carbon (ex: - represents C-C) Redraw this structure and
insert the proper number of carbons, hydrogens and lone pairs."
OH
D Add file
Based upon YOUR completed structure, predict whether it will dissolve in 10 points
water and explain why. Use a C-E-R (Claim Evidence Reasoning) format to
explain your answer.
Your answer
Based upon the structure YOU completed above, determine the molecular 3 points
formula for the compound - full credit can be earned for correctly
counting the C, H,O in incorrect structure above.
Your answer

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2


Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Mathematics, 20.04.2021 18:00

History, 20.04.2021 18:00


Mathematics, 20.04.2021 18:00






History, 20.04.2021 18:00






