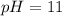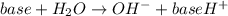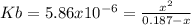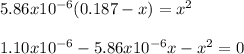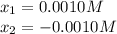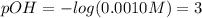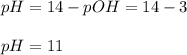Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
Do you know the correct answer?
Calculate the pH of a 0.187 M solution of a base that has a Kb = 5.86x10^-6...
Questions in other subjects:

Mathematics, 08.12.2020 16:30

Mathematics, 08.12.2020 16:30

English, 08.12.2020 16:30

Mathematics, 08.12.2020 16:30

Biology, 08.12.2020 16:30

Advanced Placement (AP), 08.12.2020 16:30


Biology, 08.12.2020 16:30

Biology, 08.12.2020 16:30