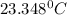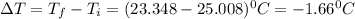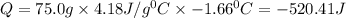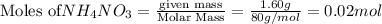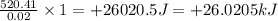Chemistry, 12.02.2021 01:40, pgfrkypory2107
In a coffee cup calorimeter, 1.60 g of NH4NO3 is mixed with 75.0 g of water at an initial temperature of 25.008C. After dissolution of the salt, the final temperature of the calorimeter contents is 23.348C. Assuming the solution has a heat capacity of 4.18 J 8C21 g21 and assuming no heat loss to the calorimeter, calculate the enthalpy change for the dissolution of NH4NO3 in units of kJ/mol.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
Do you know the correct answer?
In a coffee cup calorimeter, 1.60 g of NH4NO3 is mixed with 75.0 g of water at an initial temperatur...
Questions in other subjects:




Mathematics, 01.09.2020 22:01



Chemistry, 01.09.2020 22:01



Mathematics, 01.09.2020 22:01


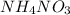 is +26.0205 kJ/mol
is +26.0205 kJ/mol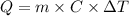
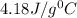
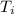 =
= 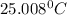
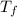 =
=